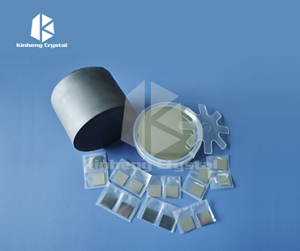
LiF Substrate
Description
LiF2 optical crystal has excellent IR performance for windows and lens.
Properties
| Density(g/cm3) |
2.64 |
| Melting Point(℃) |
845 |
| Thermal Conductivity |
11.3 Wm-1K-1 at 314K |
| Thermal Expansion |
37 x 10-6 /℃ |
| Hardness (Mho) |
113 with 600g indenter (kg/mm2) |
| Specific Heat Capacity |
1562 J/(kg.k) |
| Dielectric Constant |
9.0 at 100 Hz |
| Youngs Modulus (E) |
64.79 GPa |
| Shear Modulus (G) |
55.14 GPa |
| Bulk Modulus (K) |
62.03 GPa |
| Rupture Modulus |
10.8 MPa |
| Elastic Coefficient |
C11=112; C12=45.6; C44=63.2 |
LiF Substrate Definition
LiF (lithium fluoride) substrates refer to materials used as the basis or support for various thin film deposition processes in the fields of optics, photonics and microelectronics. LiF is a transparent and highly insulating crystal with a wide bandgap.
LiF substrates are commonly used in thin film applications due to their excellent transparency in the ultraviolet (UV) region and high resistance to heat and chemical reactions. They are particularly suitable for applications such as optical coatings, thin film deposition, spectroscopy and electron microscopy.
LiF substrates are usually chosen as substrate materials because they have low absorbance in the UV range and are optically smooth for accurate and precise measurements or observations. In addition, LiF exhibits good stability at high temperatures and can withstand multiple deposition techniques such as thermal evaporation, sputtering, and molecular beam epitaxy.
The properties of LiF substrates make them particularly suitable for applications in UV optics, lithography, and X-ray crystallography. Their high resistance to environmental factors and chemical stability make them versatile materials for various research and industrial applications.
Related Products
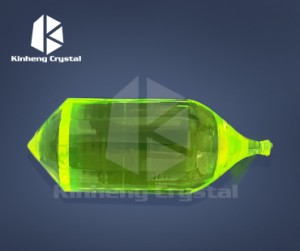
LiF (lithium fluoride) is widely known for its excellent infrared (IR) properties as an optical material for windows and lenses. Here are some key points about LiF2 optical crystals:
1. Infrared transparency: LiF2 exhibits excellent transparency in the infrared region, especially in the mid-infrared and far-infrared wavelengths. It can transmit light in the wavelength range of approximately 0.15 μm to 7 μm, making it suitable for a variety of infrared applications.
2. Low absorption: LiF2 has low absorption in the infrared spectrum, allowing minimal attenuation of infrared light through the material. This ensures high transmission and thus efficient transmission of infrared radiation.
3. High refractive index: LiF2 has a high refractive index in the infrared wavelength range. This property allows efficient control and manipulation of infrared light, making it valuable for lens designs that need to focus and bend infrared radiation.
4. Wide bandgap: LiF2 has a wide bandgap of about 12.6 eV, which means it requires a high energy input to initiate electronic transitions. This property contributes to its high transparency and low absorption in the ultraviolet and infrared regions.
5. Thermal stability: LiF2 has good thermal stability, which enables it to withstand high temperatures without significant performance degradation. This makes it suitable for applications involving exposure to high temperatures, such as thermal imaging systems or infrared sensors.
6. Chemical resistance: LiF2 is resistant to many chemicals, including acids and alkalis. It does not react or degrade easily in the presence of these substances, ensuring the long-term durability and reliability of optics made from LiF2.
7. Low birefringence: LiF2 has low birefringence, which means it does not split light into different polarization states. This property is important in applications that require polarization independence, such as in interferometry or other precision optical systems.
Overall, LiF2 is highly regarded for its excellent performance in the infrared spectrum, making it a valuable material for windows and lenses in a variety of infrared applications. Its combination of high transparency, low absorption, wide bandgap, thermal stability, chemical resistance, and low birefringence contributes to its excellent infrared performance.